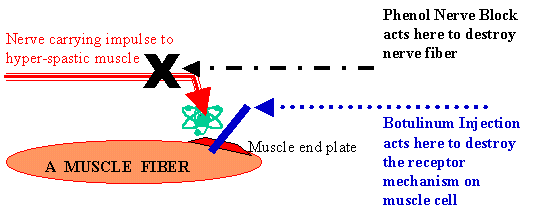
Our Experience & Comments:
The rapidity of relief from spasticity in Spastic CP with Botulinum
Muscle Infiltration Injection (which can be used a number of times) or
Phenol Nerve Block (which may be used relatively safely about twice) is
magical. However, the effects are temporary, up to 4 to 8 months in most
cases of CP.
Initially, we tried them a number of times, but have since used it rarely.
It gives so much initial relief that it takes away the drive to continue
intensive OT/PT by the child and parents. The net result in most cases is
"Back to the Pavilion" in a few months.
In contrast, tendon lengthening surgery costs
just a wee bit more than these injections, and the effects are permanent.
We prefer that if very necessary.